By interlocutory decision of 27 June 2023 in case T 438/19, a new referral G1/23 is pending before the Enlarged Board of Appeal that addresses the issue of reproducibility as a requirement for products to become state of the art. As this topic is of high relevancy in the field of chemistry, in particular polymer chemistry, we are following this topic with a heightened interest and have summarized the road to G1/23 in the following.
State of the art
The European Patent Convention (EPC) and most of the other Jurisdictions in the world employ absolute novelty when determining the state of the art for patent application. This broadest possible approach is reflected in Art. 54(2) of the EPC: The state of the art shall be held to comprise everything made available to the public by means of a written or oral description, by use, or in any other way, before the date of filing of the European patent application. (emphasis added) Thus, the only “real” limitation for suitable state of the art according to Art. 54(2) EPC is that it has to be made available to the public.
While this approach may appear prima facie straightforward, certain peculiarities regarding public availability for different forms of publications, such as commercial products, have arisen over time.
The road to G1/23
Inter alia issues concerning the amount of information from commercial products that is publically available had to be and were addressed by the Enlarged Board of Appeal in their Decisions G2/88 and G6/88. The direction that these decisions pointed at was that all information, which can be obtained by analysis of the product, is state of the art and not analysable characteristics of the product, such as interactions with further components, could in principle be patentable as a second non-medical use of the known product.
The question, if particular reasons have to be present for the skilled person to analyse a commercial product were subsequently addressed in G1/92.
While G1/92 maintained the course of the broad interpretation of state of the art by stating that no reasons to analyse a commercial product are necessary, it additionally introduced the requirement of reproducibility, although this issue was not part of the referred questions.
G1/92 Referred Question:
Is the chemical composition of a product made available to the public by virtue of the availability to the public of that product, irrespective of whether particular reasons can be identified to cause the skilled person to analyse the composition?”
Answer:
The chemical composition of a product is state of the art when the product as such is available to the public and can be analysed and reproduced by the skilled person, irrespective of whether or not particular reasons can be identified for analysing the composition. (Emphasis added)
This reproducibility requirement led to further issues, in particular in the field of polymer chemistry, as the reproduction, especially the identical reproduction of polymers is historically difficult if not impossible.
One of the most influential Decisions following G1/92 was T 1833/14, which was concerned with the reproducibility of a commercial polypropylene that fulfilled all claimed features. Here, the Board of Appeal decided that the reproduction of the commercial product with regard to the claimed features was insufficient and that it has to be identically reproducible to become state of the art. The Board further elaborated that for polymers, the type of catalyst, the reaction system and the process conditions influence the properties of the obtained polymer significantly and that these parameters have be disclosed for the product to be reproducible.
Consequently, T 1833/14 basically excluded commercial polymer products from the state of the art, since typically no information regarding the catalyst and process conditions are disclosed.
Since this Decision was included into the „Case law of the Boards of Appeal of the EPO” book and then even into the Guidelines (G-IV 7.2.1), it is often used as a general objection against commercial polymer products as state of the art.
Diverging Jurisprudence with regard to the extent of analysability (e.g. T946/04, T2458/09), degree of reproducibility (e.g. T1540/21, T1833/14) and to which part (chemical composition, internal structure or product itself) of the “irreproducible” product is excluded from the state of the art (e.g. T23/11, T1666/16) finally led to the following referred questions for G1/23, based on interlocutory Decision T438/19.
G1/23
Referred Questions:
- Is a product put on the market before the date of filing of a European patent application to be excluded from the state of the art within the meaning of Article 54(2) EPC for the sole reason that its composition or internal structure could not be analysed and reproduced without undue burden by the skilled person before that date?
- If the answer to question 1 is no, is technical information about said product which was made available to the public before the filing date (e.g. by publication of technical brochure, non-patent or patent literature) state of the art within the meaning of Article 54(2) EPC, irrespective of whether the composition or internal structure of the product could be analysed and reproduced without undue burden by the skilled person before that date?
- If the answer to question 1 is yes or the answer to question 2 is no, which criteria are to be applied in order to determine whether or not the composition or internal structure of the product could be analysed and reproduced without undue burden within the meaning of opinion G 1/92? In particular, is it required that the composition and internal structure of the product be fully analysable and identically reproducible?
Analysis of the submitted amicus curiae letters provides an insight into the general consensus, which appears to depart from the strict application of G1/92 and the reproducibility requirement.
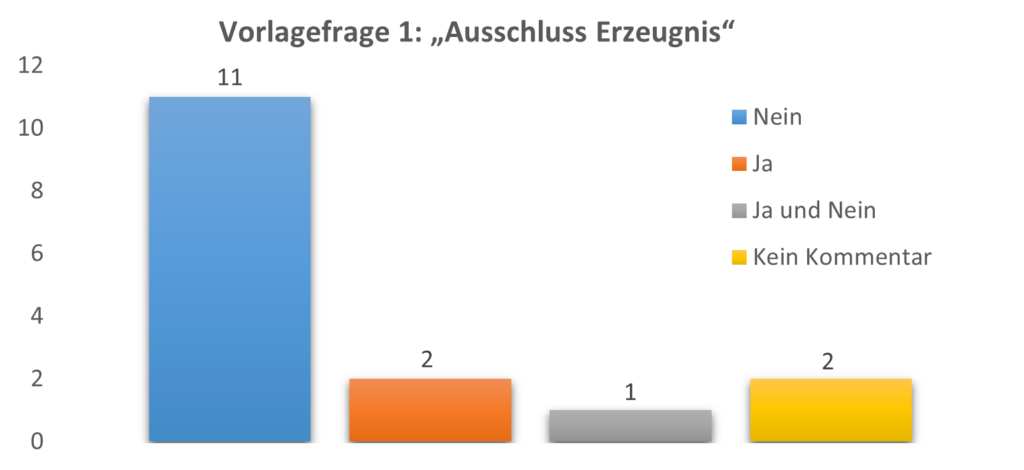
In summary, 11 parties argued against the exclusion of products from the state of the art based solely on the irreproducibility of its composition or internal structure (No to Question 1), two parties argued that G1/92 and the Case law for oral and written disclosures already provides clear guidance that irreproducible products/teaching are to be excluded from the state of the art (Yes to Question 1), one party argues that it has to be differentiated if the commercial product is state of the art for novelty or inventive step (Yes to Question 1 for novelty, No to Question 1 for inventive step) and two parties concluded that the outcome of G1/23 should only apply in the fields of chemistry and pharmacy, as the issue at hand is not present in other fields of technology.
Summary and Outlook
The recent developments in opposition and appeal proceedings, in particular in the field of polymer chemistry demonstrate that clear guidance is required whether reproducibility should indeed be a requirement for commercial products to become state of the art (Question 1) and if so, to which extent (Question 3). Depending which direction the Enlarged Board of Appeal decides to pursue, commercial polymer products might be excluded from the state of the art for good or might experience a revival. Whichever way, it will be highly interesting to analyse the hopefully extensive reasoning behind their Decision.
